The workings of medical device technology require accuracy and precision on every level, even for printed circuit boards (PCBs). When it comes to medical functions, the highest standards of assembly must be maintained.
A competent and experienced PCB Assembly Company can help fulfill these strict criteria. Blind Buried Circuits is well known for its work in medical device assembly and offers these services with unparalleled trust. Let us discuss the key standards and thoughts that can help attain professional success in this area.
Essential Considerations for Medical PCB Assembly
Making medical devices involves assembling and designing PCBs, which requires stringent care. These devices are made to be efficient and reliable and to provide the best services to the population.
The Guidelines From IPC: The Heart of Quality PCB Medical Assembly
IPC standards cover the use of PCBs in medical facilities and provide the standards needed to make the assemblies reliable and properly functioning:
- IPC-A-610 provides the performance requirements for soldering PCBs. For medical PCBs, for example, Class 3 medical-grade soldering should be used. This is the highest level of soldering reliability.
- IPC-6012: This standard sets performance and quality requirements for rigid PCBs and encompasses application and performance standards for high-reliability medical devices.
- IPC-7711/7721: This document offers advice on working on circuits that have already been built. This is very important to extend the life of the medical device.
Learn About: The Critical Impact of IPC Standards on PCB Manufacturing
ISO Certifications: Global Standards for Quality Management
ISO certifications place great emphasis on the quality management systems that PCB medical assembly companies must adopt for the optimum construction of a PCB unit:
- ISO 13485: Quality management system (QMS) for medical device manufacturers.
- ISO 14971: concerns the safety of the product over its life tree.
- The aforementioned certifications guarantee that a PCB unit is met at each level of construction.
FDA Regulations: Safeguarding Patient Health
The FDA has direct and immediate authority over the assembly of medical devices in the United States and, therefore, sets important rules for PCBs.
- Establishment Registration: registration of the assembly of PCB devices.
- Medical Device Listing: recognition for the devices produced.
- Premarket Notification (510(k)) and Premarket Approval (PMA): Ensures that medical devices considered are assumed safe and perform as intended.
- Medical Device Reporting (MDR): reporting on the safety and performance of devices on the market.
- Labeling Requirements: The devices must be properly identifiable and categorized.
Quality System Regulation (QSR)
In regard to surgical procedures on the human body, the FDA enforces criteria QSR by ensuring that their developed medical devices are expertly designed and assembled based on the information above. There is also a great need for documentation, inspection, and validation during the assembly of the PCB.
Guiding principles in the production of Medical PCBs as well as their assemblies. Nonetheless, the creation of populated medical PCBs has certain non-negotiables, and those are:
- Placing Safety at Topmost Priority
Medical devices must function without a hitch to protect the patient’s life. The safety of the device must also be taken into account through adequate insulation, proper selection of appropriate materials, and soldering connections.
- Achieving Full Compliance
A PCB assembly company with experience working with the FDA, BC, ISO, and IPC requirements will increase assembly efficiency and guarantee quality.
- Designing for Dependability
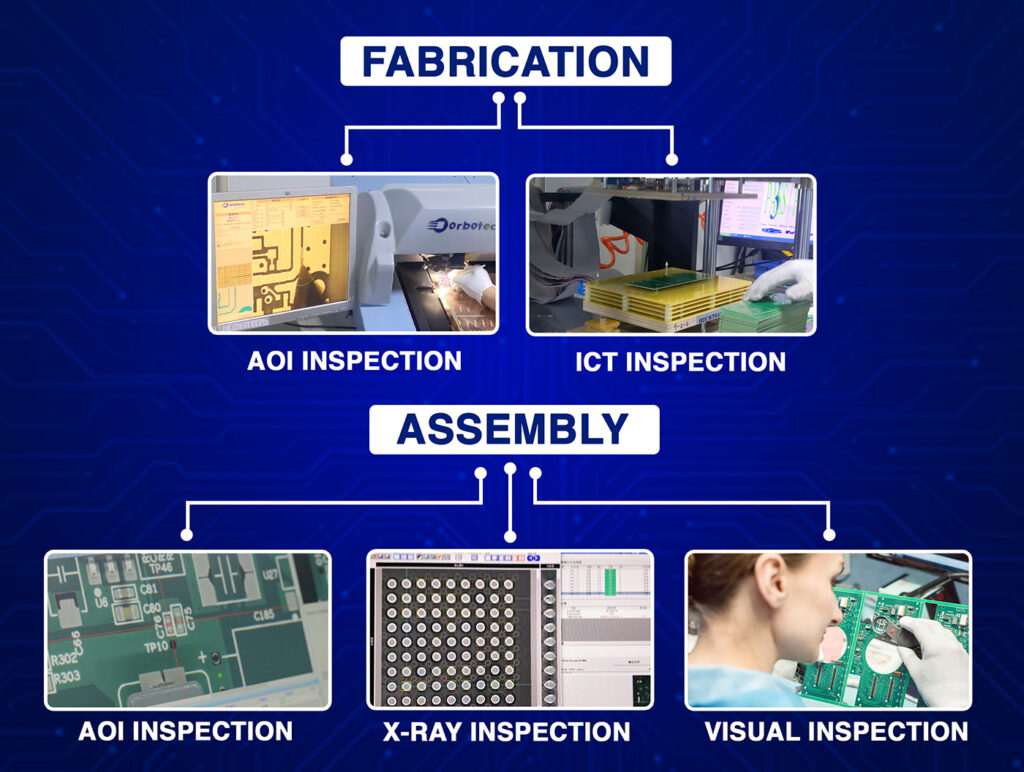
Every medical device is expected to deliver and operate without fail. Defect elimination must always include thorough testing using X-ray, AOI, and other advanced inspection methods.
- Controlling the Cleanliness of the Assembly Sites
Cleanliness during the assembly processes is essential to eliminate any contamination that might affect the quality of the medical devices.
- Working Towards Minimum Lag In Manufacturing
It is essential to provide assistance on time without delays. A responsive PCB assembly company backed up by an adequate supply chain can considerably reduce the production lag.
Extent of QMS in PCB Medical Assembly
Quality Management System (QMS)
It is important that a firm that has a strong QMS incorporates and upholds high standards from the start to the end of the manufacture through constant checking of production processes, conducting baseline tests, and enforcing regulations.
DMAIC Model for Process Improvement
DMAIC is an acronym for five stages of process improvement: Define, Measure, Analyze, Improve, and Control. This model facilitates improving the quality and productivity of PCB medical assembly processes.
HIPAA Compliance: Securing Patient Data
When implementing a medical device that captures and transfers patient data, it must heed the Health Insurance Portability and Accountability Act regulations. This includes the following steps:
- Ensuring data accuracy during PCB design.
- Facilitating secure component communication.
- Providing measures for restricting access.
Why Blind Buried Circuits is Your Trusted Partner for Medical PCB Assembly
Blind Buried Circuits is among the most critical medical PCB assembly companies. This is the reason why:
Expertise in Compliance
Blind Buried Circuits guarantees compliance with all IPC, ISO, and FDA requirements, including the most difficult ones. Thus, the client need not worry about achieving compliance.
Advanced Manufacturing Capabilities
State-of-the-art facilities support the ability to assemble even the most complex medical PCBs precisely.
Robust Quality Control
Blind Buried Circuits with AOI and other types of comprehensive testing guarantee the reliability and safety of the broadest medical PCBs.
Tailored Solutions and Collaborative Approach
The team partners with the clients in meeting their particular needs with respect to the specific medical device designed.
Meeting the stringent requirements in medical PCB Assembly entails skills and precision and, above all, full compliance with government regulations. Blind Buried Circuits can provide you with reliable and high-quality PCB assembly service. Are you ready to guarantee that your medical devices are manufactured to the highest standards? Reach out to one of the best PCB assembly companies, Blind Buried Circuits, today, and let’s begin working on your next project.